In the journals JGR: Planets and JGR: Machine Learning and Computation, BAERI Research Scientist Ariel…
The Quantum Chemistry Lab’s Biggest Questions about the Tiniest Subjects
By Rachel Sender
The Quantum Chemistry Laboratory at the NASA Ames Research Center is not a typical chemistry lab — there are no glass vials brimming with colorful chemicals, bubbling together in thrilling experimentation. Rather, this lab is a cluster of computers that can process incomprehensible amounts of information in a relatively short amount of time. They use quantum computational modeling techniques to understand the complex relationship between molecules and their environment and work to answer big questions about the origin, preservation, and fate of life in the universe.
The Lab was founded by the late Dr. Timothy Lee, a pillar in the field of astrochemistry. During Lee’s 33-year career, he was responsible for developing theories and methods that are used broadly by quantum chemists worldwide. Lee began working for NASA Ames in 1989 and focused his work on important atmospheric and astrochemistry questions that became central to the mission of the Quantum Chem Lab: what greenhouse gas characteristics cause them to trap heat? What conditions spark life? And, where else in the universe could life exist?
The people who make up the lab are a collective of researchers who spent years collaborating with Lee, including BAERI’s Partha Bera, SETI Institute’s Xinchuan Huang, and a fluctuating group of postdocs and graduate students.
From left to right: Partha Bera, Xinchuan Huang, and the late Timothy Lee of the Quantum Chem Lab. Images: NASA.
The researchers use the science of quantum mechanics, rules that govern super tiny objects like atoms, to model how a molecule made from a variety of atoms will behave, and even look, in variable environments. Bera was kind enough to have a lengthy conversation with me about the Quantum Chem Lab: its composition and research goals. Bera gave off an air of thoughtfulness and patience as he explained: “Our lab is our computers. We can find out using quantum computation the properties of [potential] molecules, meaning: will the molecule look green or blue? What will the molecule smell like? If the temperature is high, is there going to be a significant change in their property? What will a molecule millions of light-years away look like when viewed by a telescope?”
Quantum Chemistry of Greenhouse Gases
In the mid-2000s, the researchers at the Quantum Chem Lab, in collaboration with Purdue University, set out to use their expertise in quantum chemistry to understand what makes a greenhouse gas dangerous to the environment and how to create safer alternatives.
“There are thousands and thousands of molecules in the atmosphere, but only a few are culprits. Those are, of course, carbon dioxide, [and] there are others that are man-made with no natural sources. We [humans] put them in the atmosphere,” Bera said.
Greenhouse gases and global warming are not new concepts. The man-made greenhouse gases called chlorofluorocarbons (CFCs) were recognized as dangerous in the 1980s, and in 1987, an international treaty called The Montreal Protocol was passed, placing an international ban on the use of CFCs. The Protocol was successful in cutting down CFC emissions and slowing down their negative effects on the atmosphere. Other harmful greenhouse gases, hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs) were still being used, however, and safer alternatives couldn’t be designed because no one understood the underlying mechanisms that caused the gases to trap heat.
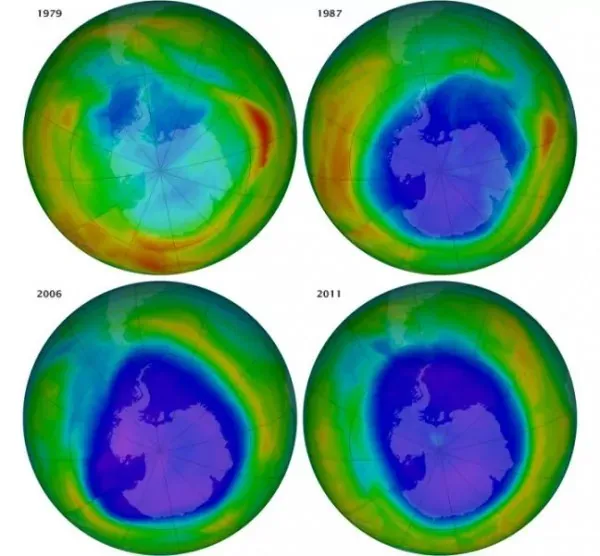
Image of the ozone hole’s expansion from 1979–2006, and evidence of some recovery from 2006–2011. Image:Earth Observatory, NASA.
Using computational modeling techniques, the researchers at the Quantum Chem Lab were able to discover the common factor of these harmful man-made gases: the high polarity of their bonds, especially carbon-fluorine bonds. These bonds can absorb larger amounts of heat than other natural compounds found in the atmosphere, like carbon dioxide, by expanding their ability to absorb infrared radiation. While many bonds leave windows in the infrared spectrum that allow heat to escape from the atmosphere, highly polarized bonds like carbon-fluorine take up more of the infrared spectrum, effectively blocking those windows and trapping in heat.
After identifying in 2009 that the highly polarized nature of carbon-fluorine bonds is what contributed to their dangerous greenhouse-gas effect, Bera and Lee published a paper in 2010 laying out design strategies for minimizing their potency. In a 2009 interview for New Scientist, Lee said, “It’s not just the number of carbon-fluorine bonds that is important, but where and how they are distributed within the molecule.” Bera and Lee showed that these chemicals could be made less dangerous by changing the location and frequency of carbon-fluorine bonds within compounds, as well as by limiting the length of carbon chains in the molecules.
While these findings were published nearly a decade ago, industry is only just now starting to work with Bera and his colleagues on implementing practices for creating safer compounds. This work is integral to the preservation of life on Earth; we know from past mass extinctions related to global warming how sensitive life is to climatic changes and atmospheric conditions.
Quantum Chemistry in Space
Reaching past our own atmosphere, Bera and the Quantum Chem Lab are also able to use modeling to understand how new life could form in space and which planets fit the criteria necessary for that to occur.
Fundamental rules, otherwise known as first principles, govern the universe. When we understand these rules, whether on the scale of tiny atoms or larger objects, hypothetical scenarios can be developed to predict potential outcomes — these are models. With the help of technology, millions of scenarios can be created and played out to determine which represents the best model for understanding the questions or processes being tested. “This is how we find out what a molecule may look like, even for molecules that have never been seen or detected before,” Bera said.
I couldn’t help but relate this kind of modeling to my own work. As a paleontologist, I use modeling techniques to infer dietary adaptations of extinct human species from information about teeth and biomechanics. In paleontology, we have only fragments of information, often muddled by millions of years of degradation, so we need to be creative to find methods that allow us to glean as much knowledge as possible from the little we have access to. Similarly, in terms of studying space, only glimpses of information make it to Earth from unimaginable distances over long expanses of time, leaving large gaps in knowledge and data. This is why theory is so important for all walks of science.
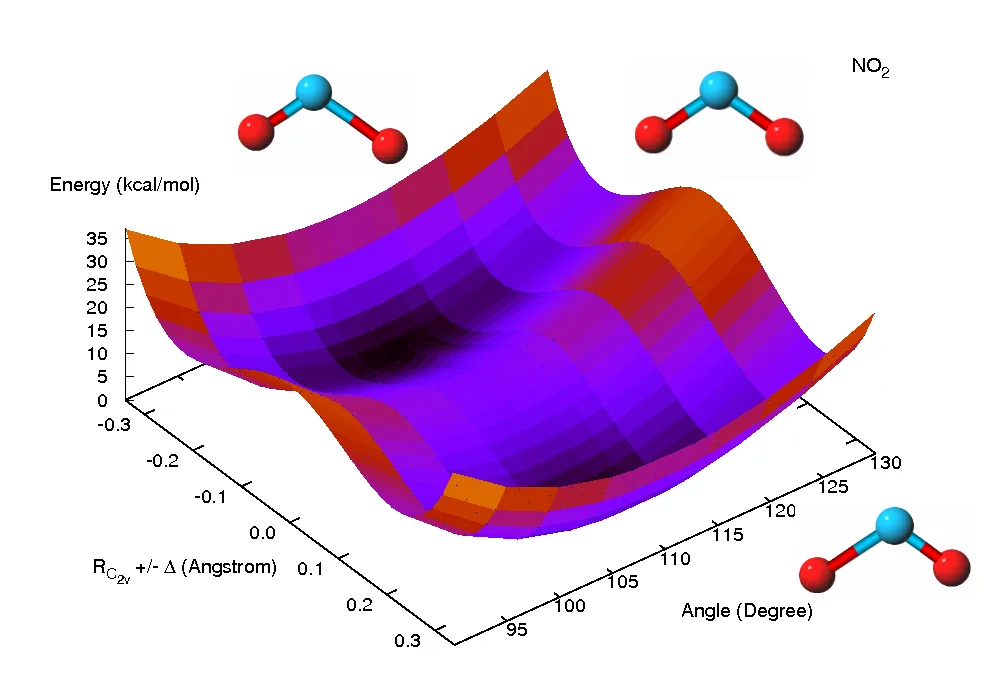
A graphic showing a model (predicting the potential energy surface of the C excited state of nitrogen dioxide) computed using state-of-the-art quantum chemistry methods. Image: Partha Bera.
“[Using models,] we try to figure out what would happen to certain molecules when they react with each other and what new molecules could be produced. Part of our work is to find out how chemistry evolves in conditions that are alien to Earth,” Bera said.
Historically, it was thought that the formation of complex organic molecules couldn’t be supported in the harsh environments of space, but this has since been proven wrong. It is now known that these types of molecules are common in space because of the abundance of materials and time — in the context of the roughly 10-million-year cycle of star formation. Stars form in molecular dense clouds, jam-packed with diverse elements ready to be combined into new and exciting compounds. As a star ages and dies, raw material is released into space. While most of the raw material doesn’t survive to travel to new clouds or planets, just enough makes it out to cause a stir and introduce new elements and compounds to faraway solar systems. We have evidence of this from meteorites that landed on Earth.

Pillars of a molecular cloud. Image: NASA.
Intriguingly, included in the material found in molecular dense clouds are what we consider the “building blocks of life” (i.e., amino acids, nucleobases (units of DNA), and sugars), and should these compounds happen to find their way to a potentially habitable planet, the possibility exists for new life to form. Now for the kicker: the diversity of these compounds found on meteors is much more expansive than those found in known living organisms on Earth. Proteins as we know them on Earth are made up of 20 amino acids, but over 70 amino acids have been identified on meteorites.
Combining fundamental principles that govern physics and chemistry, data about the many diverse molecular building blocks found in space, and variable atmospheric conditions, the lab’s computers are able to run nearly endless scenarios predicting the many potential outcomes of such alien chemical interactions. “We can predict new molecules, so this really takes the science forward,” Bera said. The Quantum Chem Lab has shown that many of the common compounds available in space from molecular clouds are likely to form an abundance of organic materials. To the delight of this sci-fi nerd, the conclusion of the paper linked above (Sanford et al., 2020) reads, “life may be relatively common where local conditions [in space] are favorable,” a statement I felt was too bold to paraphrase.
So, what does the research at the Quantum Chem Lab contribute to the understanding of alien life? Well, both the question of what can exist and also where to look for it. Using computational modeling, researchers like Bera can see the potential outcomes of molecules when they react with each other in variable environments and determine the atmospheric conditions that will best promote these interactions going biogenic, i.e., able to form life.
Again, I find it helpful to look at the Quantum Chem Lab’s research through the lens of paleontology: while researchers who study life in space make predictions of what life could exist, in paleontology we predict what did exist, and both fields use clues to deduce where to best look for such life. A great example of this type of application of theory and modeling in paleontology comes from one of my favorite books, Your Inner Fish. Paleontologist Dr. Neil Shubin knew that some sort of fish with wrist bones must have existed — a transitional creature that connected vertebral life in the ocean and vertebral life on land.

An artist’s rendition of Tiktaalik. Image: Zina Deretsky, National Science Foundation.
Shubin needed to predict where to dig for such a fossil. Astrochemists need to predict where in the universe to look for life. Both gather clues that will help them choose the best possible locations to search. Researchers at the Quantum Chem Lab are able to narrow down potentially habitable planets based on spectral data (information from light) that helps map out the composition of alien planet atmospheres, since not all atmospheres prepare a planet to host life. Shubin was able to use geologic data to narrow down his search, combing through maps and surveys for exposed rock formations of the right age. While Shubin found his wristy fish Tiktaalik in the Canadian Arctic in 2004, the search for alien life is still ongoing.
As we look backward at life’s 3.5-billion-year journey on Earth and forward to the search for life in space, it is both humbling and relieving to realize we’re not so special. Boiled down, we can all be viewed as resulting from the perfect storm of just the right amino acids ending up in just the right conditions for life to form on a giant, ancient, spherical rock, spinning around an even more ancient star. That star is just one of billions in an ever-expanding universe, one that’s booming with organic building blocks hurtling towards countless potentially habitable planets. The Quantum Chem Lab’s research teaches us that life, in one form or another, will persist.